Inclusion of a technical article in the Allergen Bureau Newsletter is benefit of platinum sponsorship for FAMS 2025. Inclusion in our newsletter in does not infer any preference or endorsement, real or implied.
As food allergens can trigger a life-threatening allergic shock in people with respective allergies, many countries have laws on the labeling of allergens on food. Allergens that are deliberately part of a foodstuff according to the recipe must be clearly and unambiguously labeled on the packaging. However, this does not cover the unintentional introduction of allergens into food due to a mix-up of ingredients or cross-contamination, which poses a major risk. For this reason, there are many commercially available detection methods for allergens in food. Most of these methods are immunochemical methods in which protein(s) from the allergen is (are) detected by binding of specific antibodies to the marker protein(s). However, there are no certified reference materials with which the capabilities of the various methods can be tested. It is therefore the responsibility of manufacturers to validate their methods accordingly and the responsibility of users to check the suitability of a method for testing a particular food.
This is usually done by spiking food with a defined amount of the allergen to be analyzed. The spiked food is extracted according to the method specifications and the recovery of the extracted allergen is determined in the test. This works well if there are no interfering effects from the matrix of the food and if unintentional contamination by the allergen of this food usually occurs just by mixing of allergen containing material with the food. However, as soon as the food is processed together with the allergen (e.g. heating), conformational changes of the allergenic marker protein can occur in the simplest case. This can lead to a loss of epitope recognition by the antibody and a reduced recovery of the allergen. Moreover, interactions between the food matrix and the allergen can occur, which can also influence the recovery of the allergen. Depending on the conditions during processing, the proteins of the allergen can be aggregated with the food matrix via physical interactions.
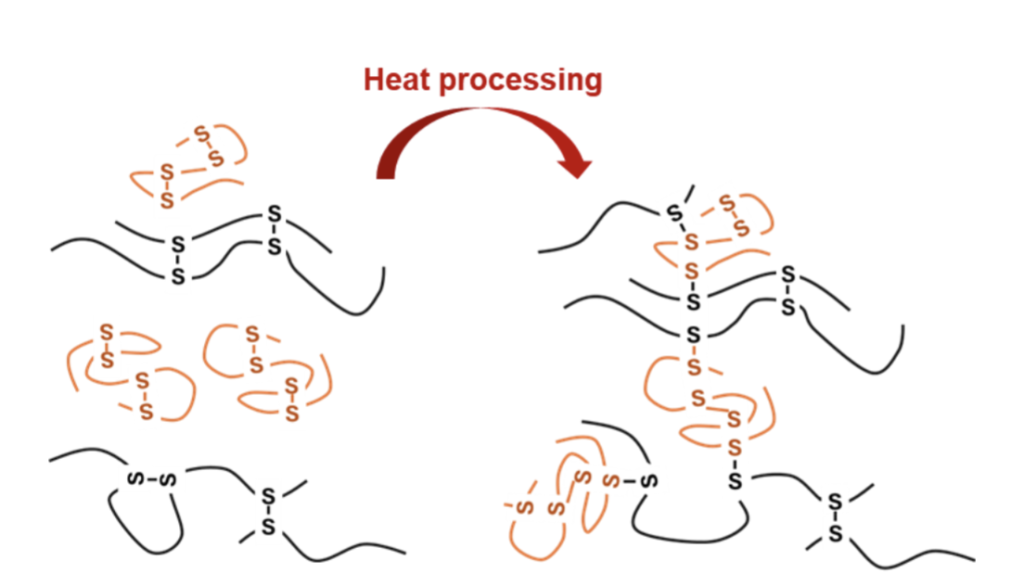
In addition, chemical covalent bonds with the matrix can also occur, e.g. disulfide bonds between the allergenic marker protein and matrix proteins can form. Thereby solubility and thus detectability of allergens can be lost. In such cases, reducing and/or disaggregating substances must be added to the food during extraction for efficient allergen recovery. E.g. Enrique Méndez developed a special cocktail solution for the extraction of gluten from heated foods [1, 2]. New links between sulfur-containing amino acids of gluten and matrix components during food production led to a reduced detectability of gluten in heated foods (Fig. 1). The Méndez-cocktail dissolves the disulfide bonds again and thus ensure better recovery of gluten.
Figure 1: Disulfide bonds changed by heat-processing in foods leading to reduced recovery of gluten
When evaluating the performance of a method for detecting allergens, it is therefore important to select suitable test material. In the past, processed foods were usually spiked after processing to determine recovery. However, as described above, this procedure has methodological weaknesses. For this reason, experts today refer to the importance of samples in which the allergens to be analyzed have also undergone all processing steps of the food, so-called ‘incurred samples’[3]. For this purpose, the ingredients must be spiked before the food is produced. Organizations such as the AOAC are revising their guidelines for the validation of methods accordingly [4, 5]. Already for the first endorsement as AOAC Official Method of Analysis™ in 2012, the RIDASCREEN® Gliadin ELISA (Art. No. R7001) was validated with incurred matrices. Following the nomination of this assay in combination with the cocktail developed by Méndez as the Codex Alimentarius Type I method, validation based on a wide variety of incurred samples was the next milestone in quality assurance in gluten analysis. Within the course of an extensive matrix extension, the RIDASCREEN® Gliadin ELISA was the first ELISA worldwide to be validated by the AOAC as an Official Method of Analysis™ based on a very wide variety of incurred samples in the year 2021. Incurred samples were used at R-Biopharm already in the past for assay validation, but the emphasis on incurred samples in the course of newly developed methods for the detection of allergens has been further increased. Examples of this are the tests in the new EASY ELISA line or the new RIDA®QUICK Gluten quant. test, a test strip for the quantitative determination of gluten in food and hygiene samples. The use of incurred samples also in the new RIDASCREEN®EASY Gluten (Art. No. RAE7071) and RIDA®QUICK Gluten quant. (Art. No. RAL7073) tests enables the metric traceability of results to the Codex method and leads to better comparability of results obtained with these methods as part of an analysis integrated into a gluten management ‘from farm to fork’.
[1] García E, Llorente M, Hernando A, Kieffer R, Wieser H, Méndez E: Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. European Journal of Gastroenterology & Hepatology 2005, 17:529-539
[2] Valdés I, García E, Llorente M, Méndez E: Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. European Journal of Gastroenterology & Hepatology 2003, 15:465–474
[3] EN 15633-1:2019 Foodstuffs – Detection of food allergens by immunological methods – Part 1: General considerations
[4] AOAC International Guidance for Validation of Quantitative Gluten Methods, Draft for Review, Octobre 2023
[5] Latimer, George W, Jr.: Appendix M Guidance on Food Allergen Immunoassay Validation. Official Methods of Analysis of AOAC INTERNATIONAL. 04 Jan 2023. https://doi.org/10.1093/9780197610145.005.013


