It’s now been four months since the launch of VITAL 4.0, and we hope organisations have been using this time to review and update their allergen risk reviews and allergen management plans. We know you will have been busy reassessing outcomes, comparing results, and making informed decisions about any necessary label changes. We hope new products have successfully launched, aligning with the VITAL Program philosophy: to Avoid, Eliminate, and Reduce cross-contact allergens to the lowest possible level, helping consumers with food allergy to make informed food choices. It is also an opportunity to remind those providing Product Information Forms (PIF) for ingredients, that the ED05 RfD change does not provide an opportunity to increase cross contact levels communicated to customers, in fact there should be no change.
For those planning label updates that affect the precautionary allergen label, we have developed a FAQ to help you with the transition. This FAQ is available on the VITAL website and appears as a pop up in VITAL Online. The FAQ is designed to cover communication considerations where the data remains unchanged, but the outcome has changed from Action Level 2 to Action Level 1 under VITAL 4.0. The Allergen Bureau remains committed to supporting industry efforts and will continue to work closely with key stakeholders. Our shared goal is to ensure that consumers understand why label changes are occurring and to encourage them to reach out to manufacturers directly with any questions.
Thank you for your ongoing commitment to the VITAL Program and to supporting safe food practices for everyone.
VITAL ONLINE SURVEY RESULTS
A big thank you to those who completed the VITAL Online Survey in July, allowing us to get a snapshot of users see the system, its value and user priorities, prior to the migration to VITAL 4.0. Whilst the response rate was modest, we had users from ANZ, Europe and Sri Lanka all contribute and we greatly appreciate the information. The survey showed that overall, most users are (over 80%) are satisfied or very satisfied with the system, experience and support, which is pleasing.
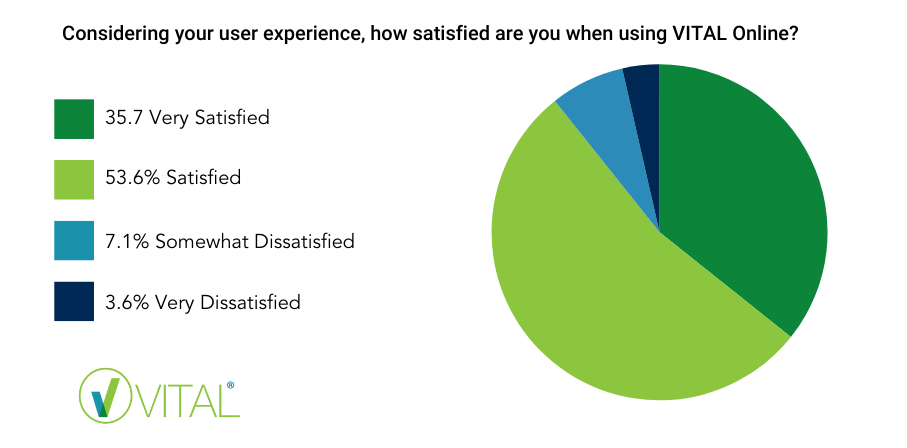
The Allergen Bureau are reviewing the feedback provided and will be prioritising the features and enhancements we deliver moving forward. Our aim is to continue to evolve the VITAL program to meet your needs, so please if you have any feedback or you would like to be involved in the super user group let us know.
We encourage all users of the system who have not attended VITAL training to consider this for your business. The VITAL tool use is underpinned by an organisation’s implementation of best practice allergen management and the completion of a robust risk assessment. VITAL training provides attendees with these skills, along with providing detailed practical training of the systems functionality. A full list of trainers can be found here (VITAL Training – VITAL)


